WHAT YOU REALLY NEED TO KNOW
ABOUT COSMETIC SURGERY
Full Text from Dr. Berman's Book
Chapter 15
AUGMENTATION MAMMOPLASTY
(Breast Augmentation)
Breast augmentation is generally one of the most gratifying procedures I perform. I have found that patients seeking breast augmentation are not simply looking to have larger breasts, but are generally interested in having nicer shaped breasts that are more proportionate with their body. Though it is true that a number of patients simply want larger breasts, this is the minority. It is amazing how many different shapes and sizes breasts may be. Well shaped and proportioned breasts can go a long way to enhance a woman's overall appearance.
In simple terms, breast augmentation is performed by creating a space under the breast tissue or even under the pectoralis (breast) muscle and then placing a silicone rubber coated implant within that space. Access to the space is achieved through a small incision under the breast, around the areola (nipple area), the axilla (arm pit) or even the umbilicus (belly button). This doesn't sound like such a big deal, but there's a lot more involved than one might expect and for this reason, it's best to start by discussing the negative aspects of the operation first.
I am aware that there is a vocal group of women who believe the breast is merely a milk gland meant for lactation. Indeed, if you look at your own breasts that way, and that makes you happy, then don't touch them. Nonetheless, there are a good many women who are much happier with fuller and shapelier breasts. Happiness, you will recall, according to the world by Berman, is one of the fundamental meanings of life. By the way, I also know several women (and not just models, actresses and dancers) who have enhanced their ability to survive (the other meaning of life) following breast augmentation.
Meanwhile, an operation that was becoming logarithmically increasingly popular, was suddenly bashed in 1992 when the FDA "pulled the plug" on the cosmetic use of silicone gel implants. Later, patients undergoing reconstructive surgery, or women with previous gel implants would become eligible for gel implants under a special investigation. Nonetheless, TV talk shows, and the media in general, picked up the cue and made breast augmentation surgery sound as though it was a dangerous scourge to our female population. Patients with perfectly fine implants, soft and beautiful breasts, became concerned, even frightened, about the potential "time bombs" sitting within their breasts. Some demanded that they be removed. And most certainly, women who had been contemplating having the procedure were now afraid to go ahead. When asked what they thought was the problem raised by the FDA, many simply said they understood that breast implants were "dangerous", and that they "cause cancer". I don't think I've encountered a single person who understood anything about "autoimmune disease". Over the last few years, the medical community has taken the opportunity to perform some of the research that should have been done long ago. The silicone implant is generally being vindicated as new reports are added to the world's literature. Patients have been gaining confidence and are returning in pre-FDA moratorium numbers.
So, what's all the fuss about? Before I attempt to explain the "fuss," let me stand on record and say that the present silicone gel implants are safe, but they are not void of problems. As such, the problems are mostly caused by the patient or the doctor (not the implant), even though a better implant design is possible. Indeed, work is in progress in an attempt to develop improved implants. For now, however, let's examine the present issues. Basically, there have been a number of patients with silicone gel breast implants who have claimed that their implants, by virtue of silicone gel leaking into their body, has caused them to come down with a serious type of autoimmune or rheumatic condition. The most common diseases allegedly caused were lupus and scleroderma. There have also been claims made to a host of so-called "human adjuvant diseases." The theory ventured forth is that the silicone gel from the implants leaches into the body, in turn stimulating the body's defense mechanisms to produce antibodies against the silicone gel. These antibodies can then turn against the body's own tissues and start an immune or inflammatory reaction. This would then be an autoimmune disease. A few law suits, in spite of a lack of scientific support, were nonetheless successful on behalf of the plaintiff's position against the implant manufacturer. The FDA in turn, under a fair amount of pressure from special public watch groups, imposed a moratorium on the use of silicone gel implants. Actually, it was David Kessler, the head of the FDA that made the decision. The FDA advisory panel of specialists (excluding plastic surgeons) recommended that the gel implants be left on the market and that the patients be given the choice with an expanded informed consent. Later, the special investigational registries were established and allowed some patients to participate in trials of silicone gel implants. Still, the typical cosmetic surgery patient was excluded from the option of silicone gel implants. Saline filled implants became the alternative allowed. Many women who were allowed to participate in the "experimental program" using the gel implants were rightly outraged. Why should a woman who had breast cancer be allowed to participate in this program and not a "normal" patient seeking cosmetic augmentation. Was it okay to experiment with their less than normal bodies? Of course not, but this is the message the FDA effectively delivered.
Meanwhile, a huge class action settlement was put together principally funded by Dow Corning in order to avoid the potential avalanche of individual personal injury law suits. In spite of the lack of scientific evidence to support wrongful injury attributed to Dow Corning, it seemed likely that juries would continue to find in favor of the plaintiff. Why, if no evidence of wrongdoing existed would they do such a thing? Basically, they felt sorry for the patient and went after the "deep pockets". Fueled by an aggressive campaign from the attorneys, many more women, many women who didn't even have real problems, enlisted their names into the class action suit. It became clear that the monetary awards were going to be split into considerably smaller parcels. The only winners were likely to be law firms with large numbers of patient/clients filing in the class action settlement. A number of women then decided to "opt out" and file their own independent suits. Dow Corning eventually declared bankruptcy, probably because the litigation got out of hand, but also because of the continued accumulation of supportive research.
Research projects, notably the Mayo Clinic Study and the Harvard Study, demonstrated that there was no significant relation between women with breast implants and autoimmune disease. The revelations that silicone, declared to be "bleeding" (slowly leaking) out of the implants and into the surrounding body tissues of the affected patients became dubious. A control study was performed where fresh cadavers without implants were examined and revealed that there was a baseline level of silicone in peripheral body tissues equal to that of women with implants. At worst, studies of gel implants reveal a higher concentration of silicone in the capsular tissue and in the nearby breast tissue, but not in the distant body tissues. Interestingly, several animal and human studies have revealed a lower incidence of breast cancer in the presence of silicone implants. It should be noted that the American College of Rheumatology has made the statement that there is no scientific evidence to link silicone gel implants to autoimmune disease.
Also, it may be worth noting that the implants today are extremely well made. The shells are strong and considered to be very low bleed shells. I note this because there was once a plethora of implant manufacturers. So, at one time there were "volkswagen" implants and "mercedes" implants. In other words, there was a broad range in quality of the products available. The FDA and the public never really looked at the individual implants and manufacturers, but rather, lumped them all into the same basket. Several years ago when Ford was having all of those problems with the Pinto, they didn't have to recall all of the cars on the road.
So, what was going on? Are the implants harmful? In short, no and yes. Let me explain. In the sense that the implants have been implicated in autoimmune disease, I don't believe there is sufficient evidence to suggest there is a problem. However, a number of women, who complained of various systemic abnormalities, reportedly felt better upon removal of the implants. So, what's the explanation? Glad you asked. This is a situation where I believe the problem lies with the doctors and the patient and not the implant manufacturer. Since the body forms a scar around the breast implant and doesn't incorporate it into the body, there exists a potential space between the body and the implant. There thus remains the possibility for bacteria to enter this potential space. Bacteria can enter via the blood stream following any systemic infection or invasive dental procedure. Once it gets around the breast implant it's in heaven. Quite frequently, a soft breast will become quite firm following such an infection. Chronic, untreated infections might manifest themselves in the form of chronic fatigue or vague discomfort. It might even cause the body to increase the scar tissue around the implant, so that a capsular contracture may occur. In many cases, the patient would return to the doctor's office requesting that the breast be softened. Many doctors would then squeeze the breast over the implant quite firmly in order to rupture the surrounding scar tissue and soften the breast. This is called a "closed capsulotomy". If an implant was contaminated, then the body would be in direct contact with the infected material and have a greater opportunity to launch an immune response while new scar tissue was forming around the implant. If the implant was ruptured, then there would be multiple clumps of infected silicone present. This could wreak havoc on the body. Several papers are now out discussing this issue. Some have shown that when the implant was removed the patient ceased to have the systemic complaints. Another study revealed that as many as 81% of patients who had systemic complaints (e.g. aches and pains, chronic fatigue, rashes, etc.) had positive cultures (bacteria present) of the implants removed.
The implications of this is clear. First, a number of women are going to come down with an autoimmune disease such as lupus or scleroderma. They will get this disease whether or not they have breast implants and not because they have implants. And second, a number of women with breast implants may develop "autoimmune-like" symptoms due to a subclinical bacterial contamination of their implants. Their symptoms may be made worse by closed capsulotomy and particularly worse if the implant is ruptured during the course of a closed capsulotomy.
Having discussed all of this, there are still plenty of things to be concerned with in the operation of augmentation mammoplasty. Such a seemingly simple procedure, has a lot of points of concern beyond autoimmune disease. Indeed, it's a shame that a political, not a scientific, decision has been made preventing properly informed women the opportunity to make their own decision. Maybe they'll get it back one day.
Prior to all of this on-going hub-bub, our main concern with augmentation mammoplasty was preventing capsular contracture. Capsular contracture is a condition where scar tissue, which always forms between the implant and the breast tissue, contracts or wraps around the implant compressing it so it no longer feels soft. With the older style smooth surface silicone gel implants the capsule contracture rate was between 40 and 50%. The newer textured surfaced implants got that rate down below 4%. Indeed, all was well in Implantville, and then the FDA came along. Silicone gel filled implants were no longer available on a universal basis. Fortunately, saline filled implants were still allowed to be used. The problems then came in a different form. Capsule contracture rates didn't change, but because saline has the viscosity of water, we began seeing a new problem - wrinkling of the breast. When a textured surface saline filled implant was placed in a thin tissue patient, the skin would ripple as the under filled implant would ripple. Overfilling of the implants, a new tear drop anatomical design, and placement of the implant under the muscle, helped minimize the problem. Nonetheless, in certain patients it appears that smooth surfaced saline implants may be the best solution to reduce wrinkling. Though there is an elevated risk of capsule formation, the over-inflated smooth surface saline implant tends to keep the tissues apart sufficiently to minimize capsule formation, thus allowing the breast to remain soft. The type of breast tissue one has determines the type of implant best suited for augmentation. The decision of which type of implant to use, and where to place it is determined on an individual basis. At this time there is no one implant or technique perfect for everyone.
Patients want to have the most minimal scar. Obviously an incision has to be made somewhere in order to introduce the implant. Most recently, the umbilical (belly button) approach has been developed. It requires creating a tunnel with an endoscope which is inserted through the umbilicus up to the breast. A smooth surfaced saline filled implant is then inflated causing the pocket to be formed by the expansion of the implant. Some very good results have been realized with this technique. However, it is the development of the pocket that is the critical part or the operation, and I find this technique not as precise as I like. The standard incision for placement was, and still is for many, the incision through the infra-mammary crease. This scar, however, is not always well hidden in the crease, and can remain discolored for a long period of time before it adequately fades. The incision around the nipple, on the areola, offers another well hidden incision site. It generally heals so well that it is imperceptible. The nice thing about that area is that if the scar heals with any pigment loss, then dermapigmentation (i.e. tattoo) can be done to color in the scar and eliminate visible traces of it. The axillary (arm pit) incision also works well. It is well hidden within a crease under the arm. It tends to stay red and sometimes gets rather inflamed around the sweat glands, but over time it usually heals quite well and of course there is no scar on the breast. My personal preference at the moment is the incision on the areola. I feel I have better control of developing my pocket, the scar is under no tension and heals rapidly and most of the time imperceptibly. The axillary scar may take months to adequately fade, making it uncomfortable to raise ones arms such as at the beach or the gym. At any rate, a discussion with your surgeon will help you determine which incision is best for you.
What about sensitivity? There exists about a 5% chance of decreased sensitivity. The development of the pocket, regardless of the incision site, is the key to nerve injury. Protecting the lateral thoracic nerves helps to preserve sensation to the nipple. Occasionally, nerves are stretched or even severed in attempting to form an adequate pocket. This would result in decreased sensitivity.
Infection may occur. Active, draining infections are very rare, although subclinical ones, as previously discussed, may have been more common than originally thought. If an implant becomes grossly contaminated and draining, then it needs to be removed. The breast is generally allowed three to six months to settle prior to attempting another procedure. In rare cases, it may be attempted to remove the implant and immediately replace it with a new one. This becomes a case of judgment. It should be noted that the breast is not a completely sterile gland as it permits contact to the outside environment from the nipple via the lactiferous ducts. Rarely, "mastitis," inflammation of the breasts, particularly at the time of breast feeding can secondarily infect the area around the implant and cause subsequent scarring and possible capsule formation. Because the implant is behind the breast gland it does not interfere with nursing, however caution is advised to be careful not to develop mastitis and to notify the doctor immediately at signs of inflammation. It is probably a good idea to be treated with antibiotics before invasive dental surgery or during serious illness where there's a chance that blood borne organisms can circulate and possibly come into contact with the prosthesis.
Bleeding, or hematoma (the collection of blood inside the breast pocket) is largely preventable by refraining from the ingestion of aspirin or products containing aspirin and by performing a careful, bloodless, dissection. Some "black and blue" bruising may simply be a result of sticking an occasional surface vessel while anesthetizing the breast. Serious bleeding would cause significant swelling, pain and discoloration necessitating treatment. It is exceedingly rare.
Although this is not a complication, implants tend to block out radiation, making mammography difficult to perform. Different filler materials are being tested which allow for better penetration of the x-ray. Nonetheless, mammograms can be performed by taking tangential views of the breast. Also, manual breast examinations can still be quite adequately performed and mammography is not meant to be a substitution for palpation. While a mammogram can pick up a very early tumor, a negative mammogram does not mean the vigil of monthly self examination can be dropped. Statistically, the survival rates for a cancerous tumor found by early palpation are the same as those found by mammogram, so it's very important to continue self examination of the breasts and not solely rely on mammography.
Suffice it to say, that in reality there are more risks in driving to the doctor's office than actually having a problem while you're there. Nonetheless, complications occur from time to time as with any procedure.
Once a good understanding of the problems associated with the operation is achieved, then it's a lot more fun to discuss the aesthetic aspects, namely what size breast is desired. This can generally be achieved by a) trying on different sized implants; b) filling a plastic bag with a quantity of water or rice (which does not spill so easily) necessary to fill out a desired bra; c) reviewing photographs of models with desired shaped breast; and d) by determining the optimal size using a particular measuring technique developed for certain "tear-drop" shaped implants. This is a fun part of the procedure. A note of caution, however. Should you desire your breasts to look a certain way and only that way, then don't pursue the operation because while your breasts will definitely be enhanced, it is impossible to determine how they will precisely look. It may take months following the surgery before the skin stretches to its final resting point. Almost all breasts that start out looking a little big and firm will tend to soften and look smaller with time as the tissues relax.
While the operation can be performed under general anesthesia, I prefer intravenous sedation and local anesthesia. It is an out-patient procedure, usually performed within 1 - 2 hours. Most patients can return to work within a couple of days, though activities should be moderated for a few weeks.
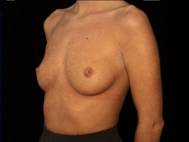
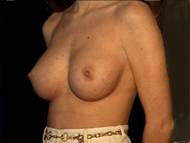
Attractive, well shaped, yet small breasts are well suited to augmentation. In this case, the implants were textured silicone rubber shells with silicone gel filling.
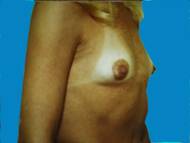
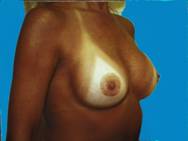
Small breasts, without much shape can be enhanced, providing contours that are more pleasing.
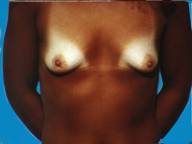
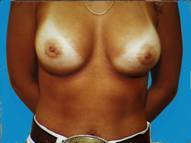
Often following pregnancy, there is a substantial loss of tissue volume which leaves the breasts appearing small and droopy. By developing an adequate pocket, implants can provide a fuller more pleasing appearance.
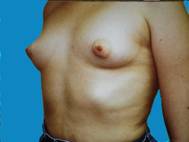
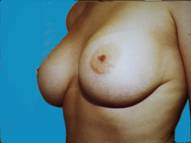
Some patients just develop small breasts and don't realize any significant growth even after pregnancy. Over the course of time, augmented breasts appear quite natural, despite some of the negative comments often ascribed to augmentation. There is no telling how much this enhances one's confidence.
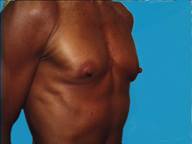
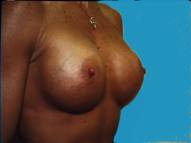
In a body builder, there is so little breast tissue that the implants generally need to be placed below the muscle to prevent serious rippling being visible through the thin tissues.
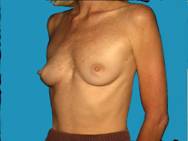
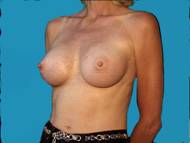
Generally well shaped breasts which have lost some volume are made shapelier with augmentation.

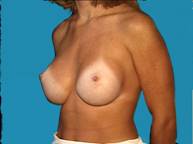
Small breasts can be difficult to dress up unless you're willing to wear padded bras or wear some type of prosthesis.
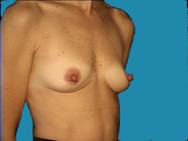

Another example of essentially nice, normal breasts that have lost some volume and are improved with augmentation.
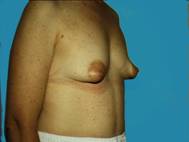
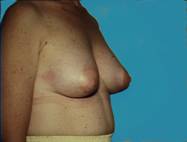
Sometimes the breast tissue is found to be very tubular (Snoopy -nose) and an augmentation procedure can provide a more normal, pleasing appearance. Most of us just want to look natural and fit in.