Breast Augmentation
Overview of Breast Augmentation and introduction to the Pocket Protector®
Breast augmentation has become one of the most popular cosmetic procedures for women. For women not well endowed in the mammary gland department, this procedure levels the playing field. For some patients, it does more than that. It may actually help them in many ways beyond simply feeling better about themselves.
The most common patient is typically one in her thirties that had a child or two and lost considerable breast volume during pregnancy or breast-feeding. They want to get back what they lost. Obviously, a number of small breast women just want to look larger or more normal. It's much more rare to see a normal shaped woman that wants to just be very large. For the most part, women desire better overall shape. Nonetheless, personal appearance is just that - personal, and as such, the key to a happy patient is to arrive at a mutually acceptable result.
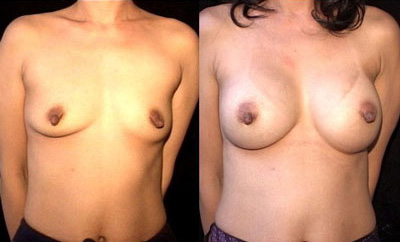
This is an example of how the Breast Augmentation with Pocket Protector
Of all the procedures I perform, this one nearly uniformly results in very happy patients. Yet, of all the cosmetic procedures available, this one has been the one laden in the most controversy. For years women had silicone gel or saline implants used to augment their breasts. Then reports started coming out about harm being caused to the patient as a result of their breast implants - particularly the gel implants. A moratorium was placed on silicone gel implants in 1992. After several years of finally investigating the problems surrounding breast implants and making improvements acceptable to the FDA, gel-filled implants have returned to the market. While gel implants were known to rupture - on their own or with applied force - sub-clinical infections were responsible for the majority of problems directly caused by implants. The implant, placed under the breast or chest muscle becomes surrounded by the body's scar tissue. We call this the capsule. While the breast implant remains trapped inside the capsule, bacteria and viruses can potentially cross the scar (capsule) tissue and enter the space around the implant. Once in there, an inflammatory reaction results and can cause further scarring thus tightening the capsule around the implant. When the implant is surrounded by capsular tissue so that it is no longer soft this is called capsular contracture. While antibiotics may temporarily control an infection, unless the implant is removed, an infected implant never gets completely cured from infectious disease. Today, we remind our patients that any infections into the blood stream can potentially seed around the implants. Perhaps the most common source for infection comes from dental intervention. For this reason, we now suggest prophylactic antibiotics prior and subsequent to invasive dental procedures.
For women augmenting their breasts for the first time, saline or gel filled implants can be selected. Gel implants feel considerably more natural. Saline can often feel a bit like a water balloon. Saline may be less prone to capsule contracture - possibly because they are firmer. Women are often concerned about the rupture of gel implants. The current gel implants are largely cohesive, however, so, if they rupture, the gel stays together and doesn't migrate into the body. In the event that a saline implant ruptures, the saline is absorbed into the body and mostly excreted in the urine. While rather benign, it would however, result in a deflation of the breast implant necessitating surgical replacement.
While Dr. Berman had developed a breast implant device – the Pocket Protector® - to help repair difficult capsule contracture, he is currently working with the FDA to try to get an approval for official testing of the device. The Pocket Protector® is made from the same material as Gore-Tex® - e-PTFE (expanded-PolyTetraFluoroEthylene) - and shaped into a bladder. It's placed under the breast or the muscle (i.e. the pocket) and serves to line that space, keeping it from scarring back down upon itself and contracting around the implant. Until he can get approval, he must put the current device on hold. Still, he can help repair the capsule problem by using two approved sheets of e-PTFE at the time of the operation to act in a similar manner to the Pocket Protector®. Until he works out an approval with the FDA for the manufactured device or some type of approval for a custom device, the actual Pocket Protector® will not be available in its current pre-operative custom manufactured form.